Phosphorylated peptide
One of the most
prominent post-translational modification (PTM) is the phosphorylation of Ser,
Thr and Tyr (Table 1). These
modifications that are easily reversible allow for the in vivo production of a myriad of proteins and thus manifold
biological fonctions from a singly translated polypeptide that varies in their
phosphorylation patterns.
Phosphorylation of
serine or threonine - and to a lesser extent tyrosine- residues prompts
conformational changes of the protein that modify their biological
activity. For example, it has long been
recognized that hormones transmit information across the external membrane of
the cells by activating transmembrane signalling systems that regulate the
production of a fairly small number of chemical mediators, called second
messengers. These messengers trigger the activities of protein kinases and
phosphatases that modify the phosphorylation states of many intracellular
proteins, thus accounting for the variety of action of hormones. Thus serine, threonine and tyrosine appear to
function as molecular switches during regulation of many cellular processes.
Phosphorylation can also be involved in
diseases, for instance the tau protein, for which abnormal hyperphosphorylation
has been related to amyloid fibril formation in Alzheimer’s disease.
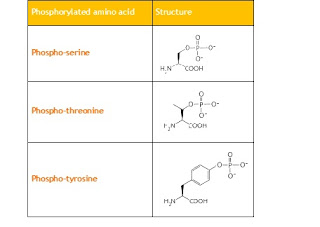
Table 1.
Chemical structure of commonly phosphorylated amino acid residues
The study of a wide
variety of biological processes often requires the use of phosphorylated
peptide to mimick natural
processes. There is a dramatic
difference between the syntheses of simple mono phosphorylated peptide and di-
or tri- phosphorylated peptide. While simple aphosphorylated peptide are
routinely synthesized using solid phase peptide synthesis (SPPS) strategies,
the synthesis of multi phosphorylated peptides remains a major synthetic
challenge. Indeed, these groups are
bulky, charged and unstable. While synthesis of phosphorylated peptides with
multiple sites of phosphorylation in close vicinity is challenging, Genosphere
Biotechnologies peptide synthesis team has been succesful in synthezing
biologically active phosphorylated peptides.
Cyclic
Peptide Synthesis
Cyclic
peptide synthesis can be used to prepare peptides that mimic natural
structures or to obtain more stable peptide analogues. The resulting cyclic peptide structure shows
enhanced conformational stability as compared to their natural
counterparts. Cyclic peptide synthesis
has been attracting considerable interest in many years.
Cyclization induces limited conformational
flexibility of the peptides. In
addition, cyclic peptides dramatically improve resistance to proteolytic
hydrolysis and degradation.
Genosphere Bioetchnologies offers on a
routine basis the two principal approaches to cyclic peptide synthesis. Ring closing may be achieved through lactam
condensation or disulfide bridge oxidation.
Basically, the lactam cycle is obtained by intramolecular amide bond
(-NH-CO-) formation between an amino group (-NH2) and a carboxyl group (-COOH).
The disulfide bridge (-S-S-) formation is
achieved between two sulhydryl (-SH) groups from the side chain of cysteines
incorporated at any position within the peptide by thiol oxidation. With a
special cyclic peptide synthesis strategy and using appropriate protecting
group chemistry to prevent unwanted cyclization, we are able to offer up to
three specific disulfide bridge.
Comments
Post a Comment